Writing IND/CTA/NDA-enabling research reports
A guide to writing research reports that enable IND, CTA, and NDA submissions.
Next Gen Editing provides gene editing, computational biology, and due diligence services to biotech, pharma, and venture capital companies to help build the next generation of medicines.
The company's principal consultant, Andrew Kernytsky, established the on- and off-target gene editing platform for the world's first company-sponsored CRISPR clinical trial, exa-cel, while leading genomics and computational biology work at CRISPR Therapeutics.
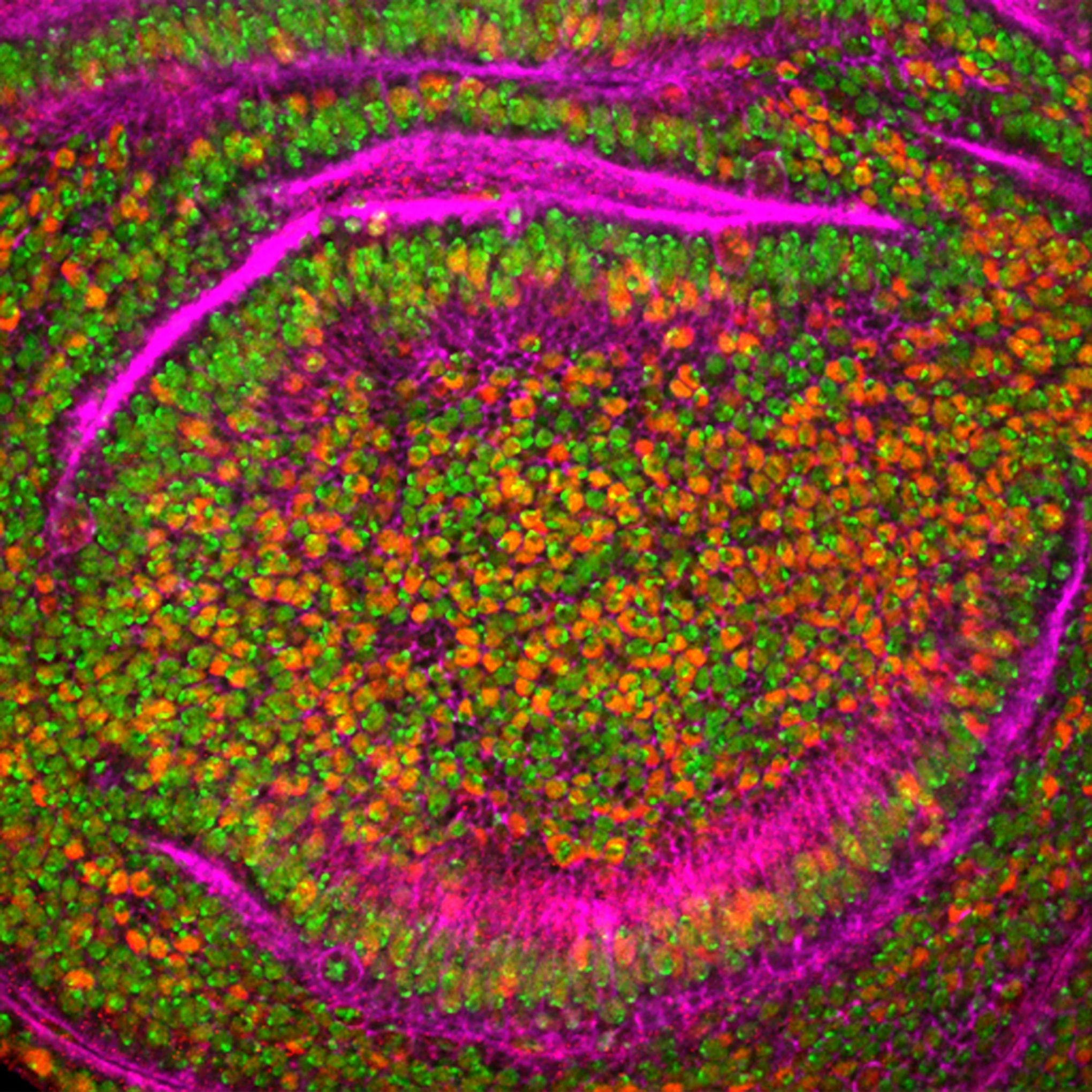
Drosophila Wing Cells: CBFA2T3-GLIS2 fusion protein (red), nuclei (green), and actin filaments (purple).
Bringing drugs from inception to the clinic is our specialty. We design and execute successful off-target analysis for all types of therapeutics.
We can help edit DNA, insert large payloads, and accurately quantify and improve the effectiveness of these changes.
Since DNA sequencing was revolutionized by Next Gen Sequencing, we've been working on the algorithms behind it. Heck, we even made it the company name!
FDA. EMA. MHRA. Hooray! Getting regulators to understand your DNA editing strategy and approve your therapy is what we're all about.
Computers and biology are our bread and butter. Peanut butter and jelly. You get the idea - we do all sorts of other analyses: epigenetics, gene expression, single cell analysis.
New venture pitches and academic projects take careful evaluation to decide whether they warrant funding. We can help evaluate and guide them.
Posts about gene editing, consulting, best practices, FAQs, investing
A guide to writing research reports that enable IND, CTA, and NDA submissions.
FAQ about off-target editing and best practices
What is the overlap between the biotech indices XBI, IBB, and ARKG?
Edit DNA faster.
Analyze data better.
Build amazing companies.Let's discuss what we can do together